Lithium-ion battery consists of positive electrode, negative electrode, electrolyte, electrolyte salt, adhesive, separator, positive electrode lead, negative electrode lead, center terminal, insulating material, safety valve, positive temperature coefficient terminal (PTC terminal), negative electrode current collector, positive electrode current collector, conductive agent, battery case and other components.
The cathode materials of lithium-ion batteries are lithium-containing transition metal oxides, phosphides such as LiCoO2, LiFePO4, etc., conductive polymers such as polyacetylene, polyphenylene, polypyrrole, polythiophene, active polysulfide compounds, etc.; lithium intercalation compound cathode materials It is an important part of lithium-ion batteries. The positive electrode material occupies a large proportion in lithium-ion batteries, so the performance of the positive electrode material will greatly affect the performance of the battery, and its cost also directly determines the cost of the battery.
At present, the research on cathode materials mainly focuses on electrode materials such as lithium cobalt oxide and lithium nickel oxide. At the same time, the rise of some new cathode materials (including conductive polymer cathode materials) has also injected new ideas into the development of cathode materials for lithium ion batteries. It is an important research content in this field to find a new system of cathode materials for lithium-ion batteries with high voltage, high specific capacity and good cycle performance.
LiCoO2 cathode material
LiCoO2 has three phases, namely LiCoO2 of a-NaFeO2 layered structure, LT-LiCoO2 of spinel structure and LiCoO2 of rock salt phase. The oxygen atoms in layered LiCoO2 adopt a distorted cubic close-packing sequence, and cobalt and lithium occupy the octahedral (3a) and (3b) positions in the cubic close-packing, respectively; the oxygen atoms in the spinel-structured LiCoO2 are ideal cubic close-packing arrangements, and lithium The layer contains 25% cobalt atoms, and the cobalt layer contains 25% lithium atoms; Li+ and Co3+ are randomly arranged in the rock-salt phase lattice, and the lithium layer and the cobalt layer cannot be clearly distinguished.
At present, LiCoO2 with layered structure is widely used in lithium-ion batteries, which has the advantages of high working voltage, stable charge and discharge voltage, suitable for high current charge and discharge, high specific energy, and good cycle performance. The two-dimensional movement between the layers of CoO2, the lithium ion conductivity is high, the diffusion coefficient is 10-9~10-7cm2·s-1, the theoretical capacity is 274 mAh·g-1, and the actual specific capacity is 140 mAh·g- 1 or so. Because of its advantages of simple production process and stable electrochemical performance, it is the first cathode material to be commercialized.
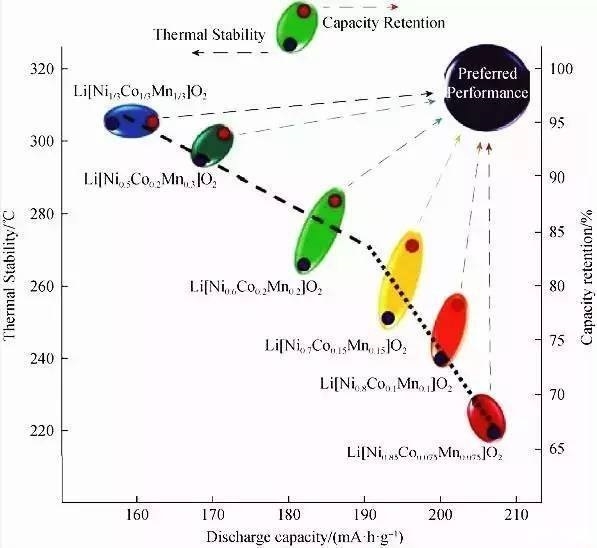
LiNiO2 cathode material
The ideal LiNiO2 crystal has a-NaFeO2 type layered structure similar to LiCoO2. The theoretical capacity of LiNiO2 is 275mAh/g, and the actual capacity has reached 190-210 mAh/g. Compared with LiCoO2, LiNiO2 has advantages in price and reserves. However, there are still many problems in the actual production and application of LiNiO2. Therefore, a lot of research has been done on the synthesis method and doping modification of LiNiO2.
The disadvantages of LiNiO2, such as difficulty in synthesis, structural phase transition and poor thermal stability, are all related to the intrinsic structure of LiNiO2. Doping LiNiO2 with elements to improve its structure is an effective means to increase the specific capacity, cycle performance and stability of LiNiO2.
Li-Mn-O cathode material
Manganese is regarded as the most promising cathode material for lithium-ion batteries due to its abundant resources, low price, non-toxic and non-polluting. There are two types of Li-Mn-O cathode materials: spinel type LiMn2O4 and layered LiMnO2.
Spinel-type LiMn2O4 has the advantages of good safety and easy synthesis, and is one of the most studied cathode materials for lithium-ion batteries. However, LiMn2O4 has the John-Teller effect, which is prone to structural distortion during charging and discharging, resulting in rapid capacity decay, especially at higher temperatures, the capacity decay is more prominent.
LiFePO4 cathode material
LiFePO4 cathode material is a new type of cathode material for lithium ion batteries. LiFePO4 is a promising cathode material for Li-ion batteries due to its abundant iron resources, low price and non-toxicity. LiFePO4 has high energy density, low price, and excellent safety, making it especially suitable for power batteries. Its appearance is a major breakthrough in lithium-ion battery materials, and it has become a research hotspot in various countries.
Conductive polymer cathode material
In lithium-ion batteries, besides metal oxides can be used as cathode materials, conductive polymers can also be used as cathode materials for lithium-ion batteries.
The currently studied polymer cathode materials for lithium ion batteries include: polyacetylene, polyphenylene, polypyrrole, polythiophene, etc., which realize the electrochemical process through anion doping and dedoping. However, the volume capacity density of these conductive polymers is generally low, and the volume of the electrolyte in the reaction system is required to be large, so it is difficult to obtain high energy density.
As the cathode material of lithium-ion battery, DMcT has advantages in specific energy, but its electrochemical redox rate at room temperature is slow, so it cannot meet the requirements of high-current discharge of the battery.


